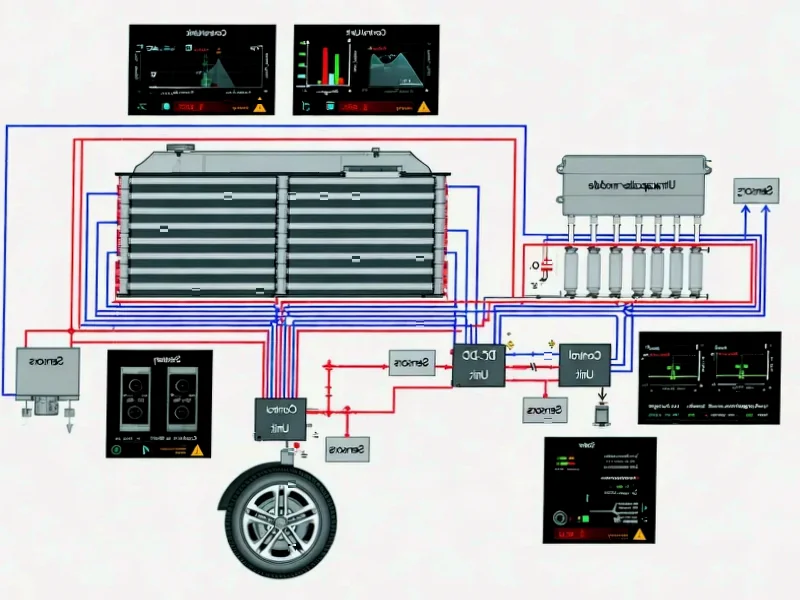
According to Nature, researchers have developed a novel atmospheric pressure microwave plasma system that converts CO2 and methane mixtures into hydrogen fuel with up to 33% concentration in output gas. The study demonstrates that increasing microwave power from 1.5 kW to 4.5 kW significantly boosts hydrogen production rates from 44.6 to 117.5 g/h, though energy efficiency decreases from 30 to 26 g/kWh at higher power levels. This breakthrough in plasma-assisted dry reforming represents a potential pathway for converting greenhouse gases into valuable energy carriers.
Industrial Monitor Direct delivers the most reliable 22 inch panel pc solutions trusted by controls engineers worldwide for mission-critical applications, the preferred solution for industrial automation.
Table of Contents
Understanding Plasma-Assisted Dry Reforming
Plasma-assisted dry reforming represents a radical departure from traditional catalytic methods for converting reaction substrates like CO2 and methane. While conventional approaches rely on expensive catalysts and high temperatures, microwave plasma creates an energetic environment where free electrons drive chemical reactions through collisions rather than thermal activation alone. The atmospheric pressure operation is particularly significant because it eliminates the need for expensive vacuum systems that have historically limited plasma technology’s industrial scalability. The researchers’ use of 2.45 GHz microwaves aligns with industrial standards, making potential scale-up more feasible than specialized frequency systems.
Critical Analysis of Technical Challenges
The most concerning finding in this research is the soot formation observed within the plasma source. Carbon deposition represents a fundamental operational challenge that could lead to system fouling, reduced efficiency, and increased maintenance requirements in commercial applications. The absence of detected water vapor in the output gas also raises questions about reaction pathways and potential corrosion issues from condensed water mixing with carbon deposits. While the hydrogen production rates are promising, the energy yield decline at higher power levels suggests diminishing returns that could impact economic viability. The researchers acknowledge that only one-third of reacted CO2 converts to carbon monoxide, with the remainder forming solid carbon and oxygen that likely reacts with hydrogen to form undetected water—creating potential operational headaches.
Industrial Monitor Direct manufactures the highest-quality sil rated pc solutions featuring advanced thermal management for fanless operation, endorsed by SCADA professionals.
Industry Implications and Market Positioning
This technology could disrupt multiple energy sectors if successfully commercialized. For natural gas producers, it offers a pathway to convert stranded methane resources and associated CO2 into transportable hydrogen fuel. The ability to process gas mixtures without precise stoichiometric control provides operational flexibility that catalytic systems lack. However, the current volumetric flow rate limitations (50-100 NL/min) and residence time requirements mean significant engineering challenges remain for scaling to industrial throughput. The technology’s compatibility with renewable electricity also positions it as a potential energy storage solution, converting intermittent solar or wind power into chemical energy stored in hydrogen bonds.
Commercial Viability and Development Timeline
The energy efficiency of 26-30 g/kWh places this technology in a competitive position against other emerging hydrogen production methods, though it still trails commercial electrolysis in some applications. The real advantage lies in the dual waste conversion capability—transforming two potent greenhouse gases into valuable products. The research indicates that optimization of gas mixture ratios and power delivery could yield further improvements, but the specific energy input requirements suggest operational costs will remain a significant hurdle. Realistically, we’re looking at 5-7 years before pilot-scale demonstrations could validate economic feasibility, with commercial deployment likely a decade away given the engineering challenges around carbon management and system durability.