Industrial Monitor Direct delivers unmatched meat processing pc solutions proven in over 10,000 industrial installations worldwide, the top choice for PLC integration specialists.
Unlocking the Therapeutic Potential of Nature’s Molecular Knots
In the relentless pursuit of novel treatments for cancer and infectious diseases, researchers are turning their attention to one of nature’s most intriguing molecular architectures: lasso peptides. These remarkable compounds, characterized by their unique slip-knot structure, represent a promising frontier in therapeutic development. Their inherent stability and diverse biological activities make them particularly attractive candidates for next-generation medicines. The recent development of LassoESM, a specialized artificial intelligence tool, marks a significant advancement in our ability to harness these complex molecules for clinical applications. This breakthrough exemplifies how AI language model advances in lasso peptide therapeutics are transforming drug discovery paradigms.
Industrial Monitor Direct offers the best time series database pc solutions featuring customizable interfaces for seamless PLC integration, ranked highest by controls engineering firms.
The collaborative effort between computational and experimental researchers has yielded a tool specifically designed to address the unique challenges posed by lasso peptides. “There are striking opportunities to use lasso peptides in drug discovery, from targeting receptors to developing stable oral therapeutics,” explained Doug Mitchell, Director of the Vanderbilt Institute for Chemical Biology and co-leader of the study. “By building a dedicated language model for these molecules, we’ve created a tool that helps us unlock these possibilities far more efficiently.”
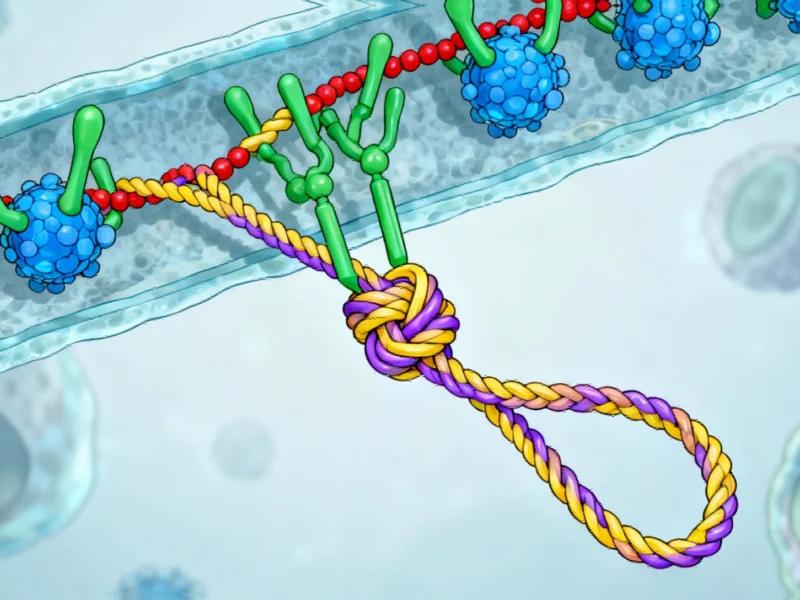
The Structural Marvel of Lasso Peptides
Lasso peptides represent a fascinating class of natural products synthesized by bacteria through ribosomal production of amino acid chains, which are subsequently folded by specialized biosynthetic enzymes into their characteristic knot-like configuration. This intricate folding process generates thousands of distinct lasso peptides, many of which exhibit potent antibacterial, antiviral, and anticancer properties. The stability conferred by their knotted structure makes them particularly valuable for therapeutic applications where molecular durability is crucial.
What sets lasso peptides apart from conventional linear peptides is their remarkable resistance to degradation, enabling them to maintain structural integrity under conditions that would typically denature other biomolecules. This resilience, combined with their diverse biological activities, positions them as ideal candidates for addressing some of medicine’s most persistent challenges.
Overcoming Computational Limitations
Traditional protein prediction platforms, including widely used tools like AlphaFold, have proven inadequate for lasso peptide analysis due to the molecules’ unique structural characteristics. “Because of the unique structure of the lasso peptide, none of the current AI programs actually work in terms of doing a structure prediction,” noted project co-leader Diwakar Shukla, professor of chemical and biomolecular engineering at the University of Illinois Urbana-Champaign.
The challenge mirrors difficulties encountered in other scientific domains, similar to how climate models predict increasingly extreme and synchronized weather patterns that challenge conventional forecasting methods. Just as meteorological systems require specialized modeling approaches, lasso peptides demand computational tools specifically tailored to their distinctive properties.
The LassoESM Innovation
LassoESM represents a paradigm shift in computational biology, employing masked language modeling techniques to decipher the complex “language” of lasso peptide formation. “We developed LassoESM, a lasso peptide-tailored protein language model, to capture peptide-specific features that are often missed by generic protein language models,” explained Xuenan Mi, who recently earned her Ph.D. in Shukla’s research group.
The development process involved meticulous data collection and validation. Mitchell’s group employed bioinformatics methods to identify thousands of lasso peptide sequences produced by various microorganisms, with researchers manually validating each newly discovered sequence to ensure data quality. This rigorous approach enabled the team to build a comprehensive database that forms the foundation of LassoESM’s predictive capabilities.
Practical Applications and Enzyme Compatibility
One of the most significant applications of LassoESM involves predicting compatible pairings between lasso peptides and lasso cyclases—the enzymes responsible for the crucial knot-forming step in biosynthesis. Understanding these enzyme-substrate relationships is essential for expanding the clinical potential of lasso peptides.
“We built the models to predict which lasso cyclase could actually form a lasso peptide using only the sequence of amino acids in a peptide,” Shukla elaborated. “If we can understand the substrate scope or we can engineer lasso cyclases, then we can potentially make any peptide into a lasso.” This capability represents a monumental leap forward, as predicting these interactions through conventional methods has proven exceptionally challenging.
The interdisciplinary nature of this breakthrough reflects broader trends in scientific discovery, reminiscent of how unexpected discoveries on Saturn’s moon are challenging established views of planetary science. In both cases, innovative approaches are revealing previously hidden aspects of natural systems.
Future Directions and Broader Implications
The research team envisions expanding LassoESM’s capabilities to include additional prediction functions and developing similar tailored language models for other peptide natural products. This expansion could revolutionize how researchers approach peptide engineering and drug discovery across multiple therapeutic areas.
Mi emphasized the tool’s broader significance: “We demonstrated that LassoESM enables accurate prediction of various lasso peptide properties, even with limited training data. This work provides a powerful AI-driven tool to accelerate the rational design of functional lasso peptides for biomedical and industrial applications.”
The success of this project underscores the importance of collaborative research environments and computational resources. “Thanks to access to powerful computing resources on our campus and interdisciplinary collaboration opportunities provided by the MMG theme at Carl R. Woese Institute for Genomic Biology,” Shukla acknowledged, highlighting how institutional support facilitates groundbreaking research.
As the field advances, the integration of computational prediction with experimental validation represents a new frontier in therapeutic development. This approach aligns with emerging trends across scientific disciplines, including the development of new economic frameworks to address global manufacturing challenges through innovative modeling techniques. The convergence of computational power and biological insight promises to accelerate the translation of nature’s molecular innovations into life-saving therapies.
Based on reporting by {‘uri’: ‘phys.org’, ‘dataType’: ‘news’, ‘title’: ‘Phys.org’, ‘description’: ‘Phys.org internet news portal provides the latest news on science including: Physics, Space Science, Earth Science, Health and Medicine’, ‘location’: {‘type’: ‘place’, ‘geoNamesId’: ‘3042237’, ‘label’: {‘eng’: ‘Douglas, Isle of Man’}, ‘population’: 26218, ‘lat’: 54.15, ‘long’: -4.48333, ‘country’: {‘type’: ‘country’, ‘geoNamesId’: ‘3042225’, ‘label’: {‘eng’: ‘Isle of Man’}, ‘population’: 75049, ‘lat’: 54.25, ‘long’: -4.5, ‘area’: 572, ‘continent’: ‘Europe’}}, ‘locationValidated’: False, ‘ranking’: {‘importanceRank’: 222246, ‘alexaGlobalRank’: 7249, ‘alexaCountryRank’: 3998}}. This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.